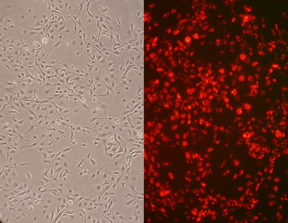
Rat Aortic Endothelial Cells (RAOEC)
电话:400-800-2947 工作微信:1843439339 (QQ同号)
邮件:Biovector@163.com
手机:18901268599
地址:北京
Cardiovascular function, studies on immune system and graft rejection, development of 3D endothelialized engineered tissues, drug discovery, stent-graft compatibility testing.
• 3rd passage, >500,000 cells in Rat Endothelial Cell Basal Medium that contains 10% FBS and 10% DMSO
• Can be cultured at least to 6th passage
Each lot was tested for proper morphology, Population Doublings, Negative for HIV, Hepatitis B, Hepatitis C, mycoplasma, bacteria, and fungi.
RAOEC from Cell Applications, Inc. provide a useful model system to study many aspects of cardiovascular function and disease. Co-culture of the artery endothelial cells with species-matched smooth muscle cells provides an ideal model for studying the interaction between these two cell types.
RAOEC from Cell Applications, Inc. have been utilized in a number of research publications to:
• Investigate critical signaling pathways and mechanisms relevant to proper endothelial function, such as angiogenesis, proliferation, permeability, and search for beneficial modulators for therapeutic use (Sorensen, 2008; Liu, 2009; Makino, 2009; Masuda, 2012; Gros, 2013; Nguen, 2013)
• Elucidate molecular mechanisms of various cardiovascular risk factors, including those associated with diabetes, polycystic kidney disease, treatment for allograft rejection and vascular ER stress (Makino, 2008; Chiasson, 2011, Ren, 2011; Padilla, 2013)
• Provide a gold standard control for endothelial markers vWF and PECAM/CD31 expression (Hanley, 2008; Imamura, 2010)
• Develop drug delivery system for thrombolytic treatment (Mei, 2010)and methods for cardiac regeneration (Lionetti, 2010); design better vascular implants (Ibrahim, 2008; Yao, 2008; Duffy, 2011) and bone tissue engineering (Hamid, 2014)
Additionally, RAOEC from Cell Applications, Inc. were used to investigate the reasons for differential effects of Intermedin (IMD) on permeability of endothelial cells from different vascular beds (Aslam, 2011). Specifically, it was shown that IMD increased permeability of rat coronary microvascular endothelial cells by inducing loss of VE-cadherin from cel-cell junctions, but it reduced permeability of RAOEC and HUVECs because in those cell types IMD instead caused accumulation of VE-cadherin in cell-cell junctions. These differential effects of IMD on VE-cadherin are explained by the fact that, although IMD activates cAMP/PKA and inhibits RhoA/ROCK pathways leading to rearrangement of actin in both cell types, it only causes downstream Rac1 inhibition in rat coronary microvascular endothelial cells, but not in RAOEC or HUVEC. These results highlight the fundamental differences between endothelial cells from different vascular beds and/or microvascular vs. macrovascular endothelia.
biological source Adult rat aorta from rat (Adult rat aorta)
growth mode Adherent
morphology Endothelial
relevant disease(s) cardiovascular diseases, diabetes
Storage
Preparation for Culturing
Culturing RAOEC
Subculturing RAOEC
Materials
A. Cryopreserved Vials (R304-05)
Store the cryovials in a liquid nitrogen storage tank immediately upon arrival.
*Be sure to wear face protection mask and gloves when retrieving cryovials from the liquid nitrogen storage tank. The dramatic temperature change from the tank to the room could cause any trapped liquid nitrogen in the cryovials to burst and cause injury.

Rat Aortic Endothelial Cells (RAOEC)
Ensure the Class II Biological Safety Cabinet, with HEPA filtered laminar airflow, is in proper working condition.
Sterilize the Biological Safety Cabinet with 70% alcohol.
Turn the Biological Safety Cabinet blower on for 10 minutes before beginning cell culture work.
Make sure all serological pipettes, pipette tips and reagent solutions are sterile.
Follow the standard sterilization technique and safety rules:
a. Do not pipette by mouth.
b. Always wear gloves and safety glasses when working with animal cells even though all the animals have
been inspected by the USDA.
c. Handle all cell culture work in a sterile hood.
A. Preparing Culture Flask
Swirl the Attachment Factor Solution (123-100) bottle a few times to form a homogenous solution. If the solution is gelled, warm up in 56°C water bath for 10 minutes and swirl the bottle to mix the solution.
Decontaminate the bottle with 70% alcohol in a sterile hood.
Add 7.5 ml of Attachment Factor Solution (123-100) to a T-75 flask (SIAL0641) and rock the flask gently to distribute the solution evenly to cover the whole culture surface.
Coat the culture ware for 30 minutes at 37°C or 2 hours to overnight at room temperature.
Remove the Attachment Factor Solution (123-100) by aspiration in a sterile hood.
The coated flask can be used immediately or stored at 4°C for up to two weeks.
B. Preparing Cell Culture Flasks for Culturing RAOEC
Take the Rat Endothelial Growth Medium (R211-500) from the refrigerator. Decontaminate the bottle with 70% alcohol in a sterile hood.
Pipette 15 ml of Rat Endothelial Growth Medium (R211-500) * to an Attachment Factor Solution (123-100) coated T-75 flask.
* Keep the medium to surface area ratio at 1ml per 5 cm2.
For example:
- 5 ml for a T-25 flask (SIAL0639) or a 60 mm tissue culture dish (SIAL0166).
- 15 ml for a T-75 flask (SIAL0641) or a 100 mm tissue culture dish (SIAL0167).
C. Thawing and Plating RAOEC
Remove the cryopreserved vial of RAOEC from the liquid nitrogen storage tank using proper protection for your eyes and hands.
Turn the vial cap a quarter turn to release any liquid nitrogen that may be trapped in the threads, then re-tighten the cap.
Thaw the cells quickly by placing the lower half of the vial in a 37°C water bath and watch the vial closely during the thawing process.
Remove the vial from the water bath when only a small amount of ice is left in the vial. Do not let cells thaw completely.
Decontaminate the vial exterior with 70% alcohol in a sterile Biological Safety Cabinet.
Remove the vial cap carefully. Do not touch the rim of the cap or the vial with your hands to avoid contamination.
Resuspend the cells in the vial by gently pipetting the cells 5 times with a 2 ml pipette. Be careful not to pipette too vigorously as to cause foaming.
Pipette the cell suspension (1ml) from the vial into the Attachment Factor Solution (123-100) coated T-75 flask containing 15 ml of Rat Endothelial Growth Medium (R211-500).
Cap the flask and rock gently to evenly distribute the cells.
Place the T-75 flask (SIAL0641) in a 37oC, 5% CO2 humidified incubator. Loosen the cap to allow gas exchange. For best results, do not disturb the culture for 24 hours after inoculation.
Change to fresh Rat Endothelial Growth Medium (R211-500) after 24 hours or overnight to remove all traces of DMSO.
Change Rat Endothelial Growth Medium (R211-500) every other day until the cells reach 60% confluency.
Double the Rat Endothelial Growth Medium (R211-500) volume when the culture is >60% confluent or for weekend feedings.
Subculture the cells when the RAOEC culture reaches 80% confluency.
A. Preparing Subculture Reagents
Remove the Trypsin-EDTA solution (T3924) and Trypsin Inhibitor (T6414) from the -20°C freezer and thaw overnight in a refrigerator.
Make sure all the subculture reagents are thawed. Swirl each bottle gently several times to form homogeneous solutions.
Store all the subculture reagents at 4°C for future use.
Aliquot Trypsin/EDTA solution (T3924) and store the unused portion at -20°C if only a portion of the Trypsin/EDTA (T3924) is needed.
B. Preparing Culture Flask
Follow instructions in Section III A and III B.
C. Subculturing RAOEC
Trypsinize Cells at Room Temperature. Do Not Warm Any Reagents to 37°C.
Remove the medium from culture flasks by aspiration.
Wash the monolayer of cells with HBSS (H6648) and remove the solution by aspiration.
Pipette 6 ml of Trypsin/EDTA Solution (T3924) into the T-75 flask (SIAL0641). Rock the flask gently to ensure the solution covers all the cells.
Remove 5.5 ml of the solution immediately.
Re-cap the flask tightly and monitor the trypsinization progress at room temperature under an inverted microscope. It usually takes about 1 minute for the cells to become rounded.
Release the rounded cells from the culture surface by hitting the side of the flask against your palm until most of the cells are detached.
Pipette 5 ml of Trypsin Inhibitor Solution (T6414) to the flask to inhibit further tryptic activity.
Transfer the cell suspension from the flask to a 50 ml sterile conical tube.
Rinse the flask with an additional 5 ml of Trypsin Inhibitor Solution (T6414) and transfer the solution into the same conical tube.
Examine the T-75 flask (SIAL0641) under a microscope. If there are >20% cells left in the flask, repeat Steps 2-9.
Centrifuge the conical tube at 220 x g for 5 minutes to pellet the cells.
Aspirate the supernatant from the tube without disturbing the cell pellet.
Flick the tip of the conical tube with your finger to loosen the cell pellet.
Resuspend the cells in 5 ml of Rat Endothelial Growth Medium (R211-500) by gently pipetting the cells to break up the clumps.
Count the cells with a hemocytometer or cell counter. Inoculate at 10,000 cells per cm2 for rapid growth, or at 5,000 cells per cm2 for regular subculturing, into an Attachment Factor Solution (123-100) coated T-75 flask.